
Market researcher Mintel and beverage specialist Flavorman talk about the evolution of sports drinks.

Market researcher Mintel and beverage specialist Flavorman talk about the evolution of sports drinks.

Opportunities in the children’s health products market.

Superfruits are still going strong in the U.S.

While agave syrup rose to popularity a decade ago as a sugar replacement, in recent years agave syrup has also come under fire from detractors concerned about the syrup’s high fructose content.

Bestevia Reb M is produced via an enzymatic conversion process using natural steviol glycosides derived from the stevia leaf.

Beverages continue to be the next frontier for marine ingredients. What progress have fish oil and algal-ingredient suppliers made?

The company has yet another new innovation: a high-quality chia oil that it says retains all of chia’s healthful nutrients due to a special production process.

Why Cargill thinks one particular star ingredient, chicory root fiber, can play a greater role in the sugar-reduction movement.

From pistachios to peas, emerging ingredients for plant-based milks are opening the category up to additional applications and consumers.

Snack makers turn to healthy claims.

When FDA finalized its new U.S. Nutrition Facts rule in 2016, one of the label’s most notable changes was the addition of a new dual-column requirement for packages containing 2-3 servings.
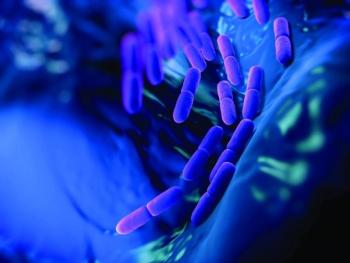
Are all fermented foods probiotic? Do probiotics delivered in a topically applied skin cream really work? Probiotics expert Ganeden takes us through five of the most common myths surrounding probiotics.
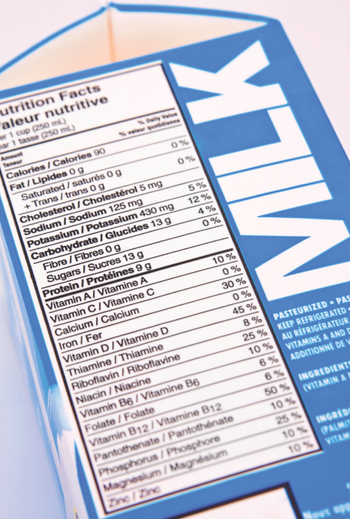
Where I do think dairy representatives have a point is when they argue that consumers could be under the impression that dairy milks and plant-based milks are nutritionally equivalent if both are called “milk.”

FDA draft guidance has given the sodium-reduction movement new life, and ingredients old and new are rising to the challenge.
Food and beverage companies in particular are of high interest to investors, said NCN.

In public comments released today in response to FDA’s recent draft guidance on dietary fiber, two industry associations told the agency that the agency’s criteria for what is considered a dietary fiber are too stringent.

Last year, the EU Commission authorized a new Article 13.5 health claim stating that when non-digestible carbohydrates are used as sugar replacers, these ingredients can induce a lower postprandial blood glucose rise compared to food and drinks containing sugar.

Ganeden says its proprietary probiotic strain is now the first and only spore-forming probiotic allowed to be used specifically in infant formulas.

Researchers in Scotland have announced plans to develop more efficient and cost-effective methods of producing natural blue colorant from spirulina algae.

Innova Market Insights reports that fruit-based snacks are now the number-three subcategory within snacks overall when it comes to new product development.

BASF says its new 10 CWD/O Plus offers a brilliant orange color that may replace azo dyes yellow 5 and yellow 6 in a variety of product types.

The joint venture will initially focus on producing ingredients from the larvae of the Black Soldier Fly, but plans to diversify into mealworms as well.

According to supplier ADM/Matsutani LLC, Fibersol-DLQ is for formulators who want to make a fiber claim but without including a “high percentage of fiber.”

TerraVia's AlgaVia algae portfolio is now approved for food use across North America, the company says.

According to Evolva, however, any plans for EverSweet are still contingent upon ongoing negotiations between Evolva and Cargill, which Evolva said it hopes to complete by the spring of this year.