
After six years of discussion, the Codex Alimentarius Commission, part of the FAO/WHO that develops international food standards, is close to finally adding a fish oil standard to the Codex Alimentarius.

After six years of discussion, the Codex Alimentarius Commission, part of the FAO/WHO that develops international food standards, is close to finally adding a fish oil standard to the Codex Alimentarius.
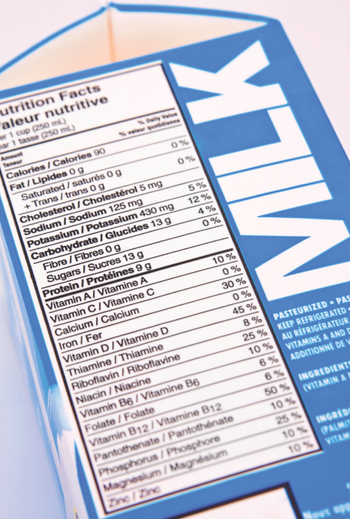
Where I do think dairy representatives have a point is when they argue that consumers could be under the impression that dairy milks and plant-based milks are nutritionally equivalent if both are called “milk.”

While there are many unknowns, the consensus seems to be that if there is any one area where the president’s policies could directly impact the industry, it is the global sourcing of ingredients.

According to the association, “this is a must-attend event for those operating in China or those thinking about it.”

FDA draft guidance has given the sodium-reduction movement new life, and ingredients old and new are rising to the challenge.

In public comments released today in response to FDA’s recent draft guidance on dietary fiber, two industry associations told the agency that the agency’s criteria for what is considered a dietary fiber are too stringent.

A new bill introduced in the New York State Assembly seeks to prohibit the sale or distribution of creatine to minors.

NPA has formed a partnership with the Informed-Choice testing program to expand the reach of its efforts to test for banned substances in U.S. supplements for athletes.

The Institute for Natural Medicine says it aims to “support the advancement of naturopathic medicine” by increasing public awareness through its new ambassador program.

On February 6, the Hemp Industries Association announced it had filed a petition for a motion of contempt against the U.S. Drug Enforcement Administration (DEA), alleging that the agency is unlawfully trying to regulate hemp food ingredients as Schedule I drugs.

A new FTC enforcement policy means homeopathic products may be harder to market going forward.

That empty space in your chip bag or other product packages? It may serve a purpose, but that might not stop consumers from suing you.

CRN also shares plans for a six-month media outreach campaign to reach its goal of including tens of thousands of finished-product supplement labels in the registry.

The SSCI includes GNC, Walmart, Vitamin Shoppe, and Whole Foods as its founding members.

Regulatory controversy may continue to follow hemp CBD, vinpocetine, and kratom this year.

The U.S. Preventive Services Task Force recommends women who may become pregnant supplement with folic acid to help prevent fetal neural tube defects.

In 2017, probiotic-industry leaders are taking steps to protect their market position by ensuring that bad actors aren’t misleading consumers with mislabeled products.

SSCI’s founding members are GNC, Walmart, Vitamin Shoppe, and Whole Foods.

Also yesterday, more 130 U.S. food and agriculture industry representatives sent a letter to President Trump specifically asking the President to protect the opportunities of NAFTA.

From the Trump administration to state attorneys general and the FDA/FTC, here are the key regulatory issues industry leaders are watching in 2017

Tensions between dairy proteins and plant proteins are heating up, but which category stands to gain the most in 2017?

In a letter sent to President-Elect Trump this week, NPA details top priorities for the supplement industry, including maternal and child nutrition, regulating NDIs, enforcing against kratom products, and more.

As Donald Trump is sworn in as President of the United States next week, Nutritional Outlook asks industry experts how his presidency could change the face of dietary-supplement contract manufacturing.

The supplement product’s extensive marketing campaign for memory benefits is based on “false and unsubstantiated claims,” according to the FTC.

The new guidelines propose a multitude of best practices for the labeling, storing, and stability testing of dietary supplements and functional foods that include probiotics.

“It is critical for us to be proactive immediately in the new year rather than reactive,” says NPA's Dan Fabricant.

According to Kemin, ZeaOne is the first dietary (3R,3’R) zeaxanthin ingredient derived from marigold flowers with an FDA-acknowledged GRAS notification.

Foods and beverages, including bakery, nutrition bar, and cereal and pasta products, can now use the claim.

In a letter to FDA last week, 32 members of Congress asked the agency to find some other name for non-dairy milks.

Eric Steenstra, executive director of the Hemp Industries Association, says the news that the DEA is creating a new drug code for marijuana extracts has “been blown completely out of proportion.”