
Maritech fucoidan ingredients are the first and only high-purity fucoidan extracts to achieve GRAS status, says the company.

Maritech fucoidan ingredients are the first and only high-purity fucoidan extracts to achieve GRAS status, says the company.

The NPA claims that FDA’s labeling changes would overly burden the food and supplement industries, and says that the new regulations around added sugars are duplicitous and that the agency’s new definition of dietary fiber is not backed by scientific or empirical studies.

GAO found that, of 490 memory supplements identified in its report, 28 of those supplements featured advertisements claiming to treat or prevent memory-related diseases-claims that are generally prohibited by federal law.

The cooperative event will include discussions on the benefits and importance of dietary supplements and functional food products, as well as the economic impact of the industry, among other key issues.

This extension is a relief to food, drink, and dietary supplement companies facing the original, fast-approaching compliance date of July 26, 2018.

Natural-ingredients supplier Jiaherb Inc. is now promoting its new HerbaLink chain-of-custody company program designed to provide its clients with traceability and transparency in product identification.

The authors conclude that short- and long-term creatine supplementation in dosages up to 30 g/day for up to five years has no adverse side effects, improves athletic performance, and can potentially help prevent injury and promote recovery.

Kemin Industries announced that after an extensive review by an independent panel of experts, the water-extracted spearmint ingredient can now be used in a variety of functional food and beverage applications, per FDA safety criteria.

Lallemand Health Solutions announced that its ProbioKid probiotic formula is now Generally Recognized as Safe (GRAS) in the United States and Canada.
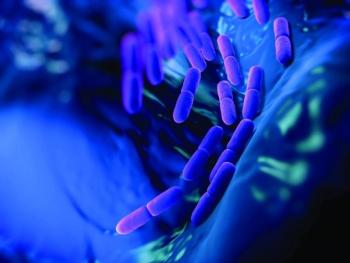
Deerland Enzymes & Probiotics’ genome-sequenced probiotic Bacillus subtilis strain DE111 has been given a Natural Product Number (NPN) by Health Canada.
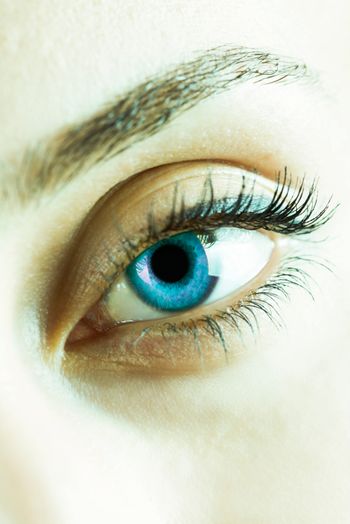
The expert panel evaluated the eye-health ingredient in accordance with FDA guidelines and found that it met the safety criteria.

The event is part of an ongoing series of conferences and seminars covering sports nutrition, probiotics, and omega-3s.

Health Canada approves Rhema Health’s Curcumin BDM30 targeting joint pain and inflammation.

Researchers found that most fish oil products on the market in New Zealand do meet regulatory limits for oxidation and label claims about EPA and DHA content.

Today the U.S. Senate voted 57–42 to confirm Scott Gottlieb, MD, as FDA Commissioner.

The government’s official definition of healthy has not been updated for more than 20 years.

NPA and CRN now officially support Gottlieb’s nomination as FDA Commissioner, with Fabricant calling for Gottlieb's “swift confirmation.”
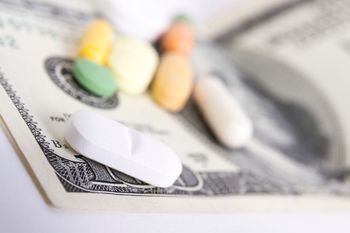
FDA’s latest request for comment regarding Current Good Manufacturing Practices in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements, released April 19, “vastly understates” the industry's expenditure, says an NPA press release.

The process patent lets Applied Food Sciences create a highly water-soluble energy ingredient.

Experts discuss emerging adulterants, strategies for catching adulteration, and whether the Designer Anabolic Steroid Control Act has made a difference.

Mythocondro, a non-animal-sourced chondroitin sulfate ingredient from Gnosis S.p.A. (Desio, Italy), has received a “no objections” response from FDA for Generally Recognized as Safe (GRAS) status for safe use in food and beverage.

The American Herbal Products Association (AHPA; Silver Spring, MD) has adopted a new guidance policy specifically to assist its members in complying with label regulations for products containing fungal ingredients.

Nearly one-quarter of the 59 new dietary ingredient (NDI) notifications companies submitted to FDA between November 2015 and October 2016 were acknowledged without objection by FDA.

Bestevia Reb M is produced via an enzymatic conversion process using natural steviol glycosides derived from the stevia leaf.

In a recent survey of Vitafoods Europe attendees and exhibitors, those surveyed said that stricter EU regulations are their top concern-even more so than the state of the global economy.

Sabinsa Corp. now holds a U.S. patent for the company’s shelf-stable Bacillus coagulans MTCC 5856 probiotic ingredient, brand name LactoSpore, for therapeutic management of irritable bowel syndrome (IBS).

At March's Natural Products Expo West trade show, Cargill (Minneapolis) announced major moves aimed to demonstrate the company’s growing commitment to non-GMO traceability.

Steve Mister, president and CEO of dietary supplements association the Council for Responsible Nutrition, told Nutritional Outlook that his organization is “optimistic” that Gottlieb will walk a “middle ground” on regulating the supplements industry.

How the European Union’s new novel food regulation aims to quicken time to market and boost innovation.

When FDA finalized its new U.S. Nutrition Facts rule in 2016, one of the label’s most notable changes was the addition of a new dual-column requirement for packages containing 2-3 servings.