
The international food standard safety authority, Codex Alimentarius, has adopted the Framework for Steviol Glycosides, which encompasses four technologies used in the production of steviol glycosides.

The international food standard safety authority, Codex Alimentarius, has adopted the Framework for Steviol Glycosides, which encompasses four technologies used in the production of steviol glycosides.

Nutritional Outlook’s Editorial Advisory Board shares its predictions and tips for success in 2022.

A more active and aggressive FTC, as well as scrutiny from other regulators, will keep dietary supplement and nutrition brands on their toes in 2022. Here’s what one lawyer says you should watch out for.

HempFusion Wellness Inc. has announced that its proprietary hemp-derived CBD extract has achieved self-affirmed GRAS (Generally Recognized as Safe) status.

FDA announced in a letter of enforcement discretion, that it does not intend to object to the use of certain qualified health claims that pertain to magnesium consumption and the reduced risk of high blood pressure.

Dietary supplement industry leaders and associations share their 2022 regulatory predictions.

In its letter, CRN takes issue with FDA’s “tentative response” which side-stepped any legal issues raised in the citizen petition.

The Federal Government of Malaysia now recognizes NSF Shanghai’s dietary supplements GMP certification (NSF/ANSI 455-2 GMP) as a requirement for Chinese manufacturers seeking to export supplement products into the Malaysian market.

The BRC standard provides a framework for the management of product safety, authenticity, quality, and legality, rating companies on a scale from AA to D.


Recent NAD cases demonstrate that brands must stay vigilant or risk regulatory action.

The China National Intellectual Property Administration issued Patent No. ZL 2017 1 1177638.2 for Certified Nutraceuticals’ Type I, Type II, Type V hydrolyzed jellyfish collagen: KollaJell.

In a recent op-ed, Marielle Weintraub, PhD, president of the U.S. Hemp Authority, warns stakeholders of the hemp and CBD industry about the importance of using trustworthy certification programs.

GRMA has partnered with IPA to establish a Probiotics Working group that will harmonize GMP requirements for probiotic dietary supplements.

The rule, issued by OSHA, requires all employers with more than 100 employees to mandate that their workers are fully vaccinated against COVID-19 or to require weekly testing and masking of unvaccinated employees.
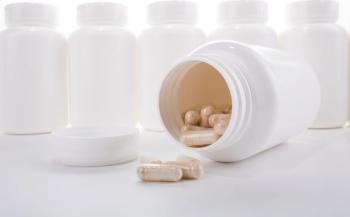
An expert reviews the important compliance checks needed to ensure the quality of dietary supplements during packaging.

The Statement of Commitment provided by NSF demonstrates and legitimizes a continued commitment to quality, compliance, and safety.

Stratum Nutrition and its Indian partner, Zenova Bionutrition Pvt. Ltd., were granted pre-market approval of its NEM brand partially hydrolyzed chicken eggshell membrane powder from the Food Safety Standards Authority of India

The Natural Products Association filed suit against FDA on December 6, calling FDA’s action to “retroactively” apply the drug-exclusion clause of the Food, Drug, and Cosmetic Act in order to ban N-acetyl-L-cysteine (NAC) unlawful.

Health Canada has approved an amendment to a Controlled Drugs and Substances Dealer’s License the agency issued to Nutrasource Pharmaceutical and Nutraceutical Services earlier this year.

Cosun Beet Company, Ingredion Inc., Matsutani Chemical Industry, and Samyang Corp. have formed a new consortium designed to help bring allulose to the European Union and United Kingdom, and support its nutritional labeling as a carbohydrate.

FDA has issued a “tentative response” to a citizen petition submitted by CRN for the agency to reverse its decision to prohibit manufacturers from marketing products containing N-acetyl-L-cysteine (NAC) as dietary supplements.

President Joe Biden has announced his nomination of Robert Califf, MD, to serve as the commissioner of the Food and Drug Administration.

The patent claim is stated as a “method of increasing GLP-1 levels in a subject in need thereof, comprising administering a composition comprising eriocitrin to the subject.”

At this October’s SupplySide West trade show, Nutritional Outlook interviewed Kyle Einhorn, HempRise’s vice president of sales, about why the company felt now is the right time to make this significant investment and about what its plans are as the U.S. dietary industry waits to find out whether hemp cannabidiol (CBD) will be made a legal dietary ingredient in the U.S.

The patent is for improving endurance and reducing fatigue during workout for pre and post workout product applications.

The new bill is a companion to Senate bill 1654.

NSF International created an introductory “GMPs for Dietary Supplements – 21 CFR 111 Overview” eLearning module, and with the promo code “DSHEA27” (from Oct 25 – 31, 2021), participants can access it at no cost.

The National Organic Program, managed by the U.S. Department of Agriculture, granted organic certification to BGG World and its subsidiary Algae Health Sciences for its astaxanthin supercritical CO2 oleoresin ingredient, AstaZine.

The Global Retail and Manufacturer Alliance (GRMA) named two new board members, Holly Korteway from Perrigo and Jeff Brams from Garden of Life.