Vedic Lifesciences is a contract research organization based in Mumbai, India. We recently analyzed trends in clinical trials on conditions associated with blood sugar. We’re providing this in-depth assessment of 83 studies related to blood sugar, glucose, and diabetes. Our analysis outlines key trends in trial design, including the demographics of study participants, study duration and sample size, and types of tools being used to conduct these studies.
To conduct our analysis, we examined the clinical trials posted over the last six years on clinical trial registry www.clinicaltrials.gov whose outcome measures related to blood sugar. The keywords we searched for were [start ital] diabetes, sugar, and glucose [end ital]. Our decision to analyze a clinical trial registry was made because a clinical trial registry provides more current data, including data on studies that are not yet published but that are in progress.
Our findings should interest a broad range of industry professionals, including: 1) clinical trial managers, chief scientific officers, medical directors, and personnel involved in designing or executing clinical studies; 2) marketing and communications managers who need to identify new studies to support their customer needs; and 3) CEOs, general managers, and senior directors who influence decisions about study design and research budgets.
Limitations of our research
- Using advanced search filters, we searched study titles for the keyword diabetes or sugar or glucose . Studies not containing at least one of these keywords in the title are not covered in this report.
- We have selected only industry-based studies and not studies funded by universities or government entities.
- We limited our search period to five years of data, from February 2018 to February 2023.
- Note that not every company registers its clinical trial on www.clinicaltrials.gov. Some companies do not register their studies in a clinical trials registry at all, or some companies register their clinical trials on other clinical trial registries, such as the ISRCTN registry. Therefore, any trials not registered on www.clinicaltrials.gov aren’t included in our analysis.
Filters Applied
Here are the filters we used in our analysis:
- Study date range: Studies registered between February 2, 2018, and February 2, 2023 (a 5-year period)
- Search terms: diabetes or sugar or glucose
- Intervention filter: Dietary supplement
- Funder type: Industry
Key Takeaway
Our analysis reveals a dynamic landscape of trends within blood sugar–management studies, emphasizing the need for tailored approaches in study design and execution. Researchers should consider the diverse biomarkers available for assessment and align these with specific study objectives to achieve more meaningful trial outcomes.
Study Status
Figure 1 indicates the status of all of the 83 studies included in our analysis.
Gender Distribution
Which populations do researchers target while designing blood sugar studies? The gender of volunteers does not appear to play a major role. As shown in Figure 2, most clinical trials for blood sugar–related claims include all genders because study sponsors want to be able to claim efficacy in a generalized population. However, while blood sugar disorders are not vastly different in men and women, certain factors impact women specifically, such as the status of postmenopausal bone health, enhanced recovery after surgery for caesarean section, or neonatal blood glucose status.
Population
These are some of the population types that blood sugar studies address:
- Pre-diabetes
- Diabetic (Type 1 & 2)
- Healthy adults
- Obese/overweight
- People with autoimmune diseases
- Juvenile/Neonatal diabetes
- Dysglycemic individuals
- People with IBS symptoms
- GI-symptomatic individuals
Vedic Lifesciences recommends a nuanced approach for supplement studies within the dynamic landscape of blood sugar management research. Specifically, consider directing your focus towards prediabetic or borderline-diabetic individuals to enhance the effect size and relevance of your findings. This strategic adjustment, rather than exclusively targeting completely healthy individuals, can offer a more comprehensive understanding of a supplement’s efficacy and effect size.
Key Strategy
Precision Demographic Targeting: Concentrate on individuals at the prediabetic or borderline-diabetic stage who have heightened sensitivity to the intervention, potentially magnifying observed effects.
Confirmation Tests: To validate the prediabetic status of participants, Vedic Lifesciences advises the inclusion of essential diagnostic tests:
- Fasting Blood Glucose
- HbA1c (Glycated Hemoglobin)
- HOMA-IR (Homeostatic Model Assessment for Insulin Resistance)
Study Duration
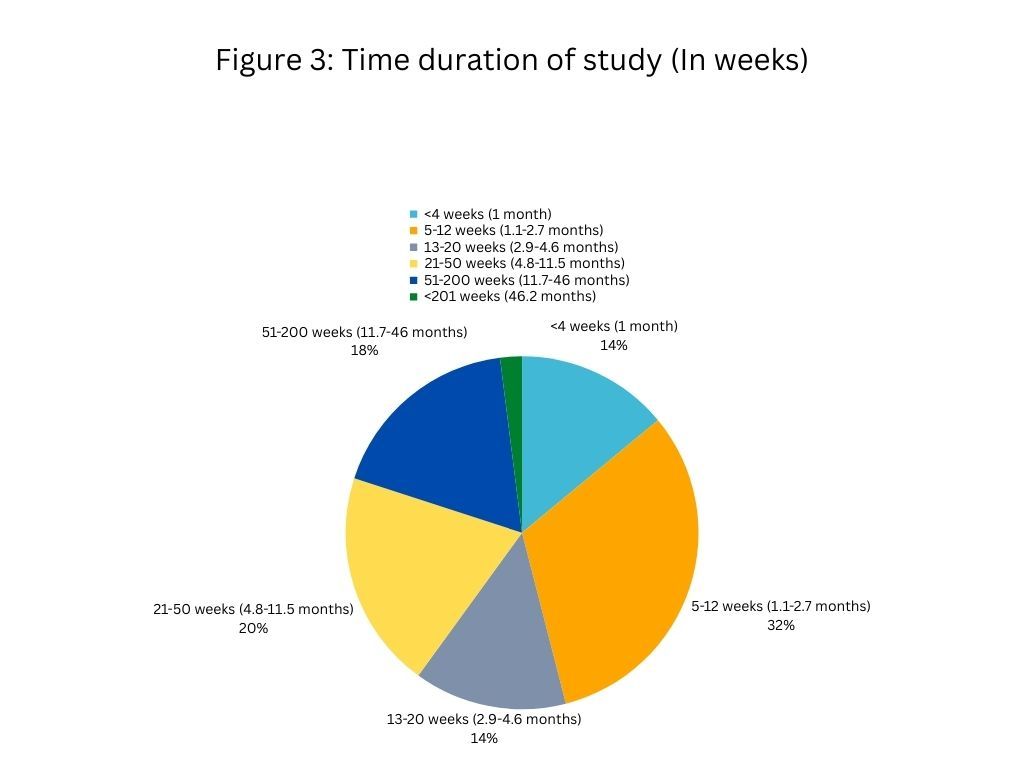
Figure 3 illustrates how blood sugar studies differ in terms of duration. Most studies had a duration of 5-12 weeks. The second most common duration was 21-50 weeks. Cumulatively, more than 50% of the total studies had a duration of under 6 months.
Longer treatment durations are ideal in that they often yield more substantial effect sizes; however, the commercial aspirations of brands can impact the decision of how long a study should be. Ideally, a more extended treatment duration allows for a comprehensive evaluation of a product’s impact on blood sugar management. This extended observation period facilitates a thorough understanding of sustained effects and potential long-term benefits. Market dynamics, however, sometimes demand quicker substantiation of health claims. Brands are often inclined to demonstrate efficacy within shorter time frames to align with consumer expectations and to capitalize on immediate market opportunities.
Studies in the 5-12 week range are most common in blood sugar research. This duration, spanning 1.1 to 2.7 months, strikes a balance between the opportunity to acquire meaningful insights and meeting the constraints of commercial timelines.
This delicate balance between scientific rigor and market urgency presents both challenges and opportunities. Researchers must navigate this landscape strategically, ensuring studies align with industry expectations while maintaining scientific integrity.
Brands and researchers must consider the strategic positioning of their studies. Shorter studies serve as initial validations, while longer-term investigations may follow to bolster claims and enhance the product’s market standing.
Sample Size
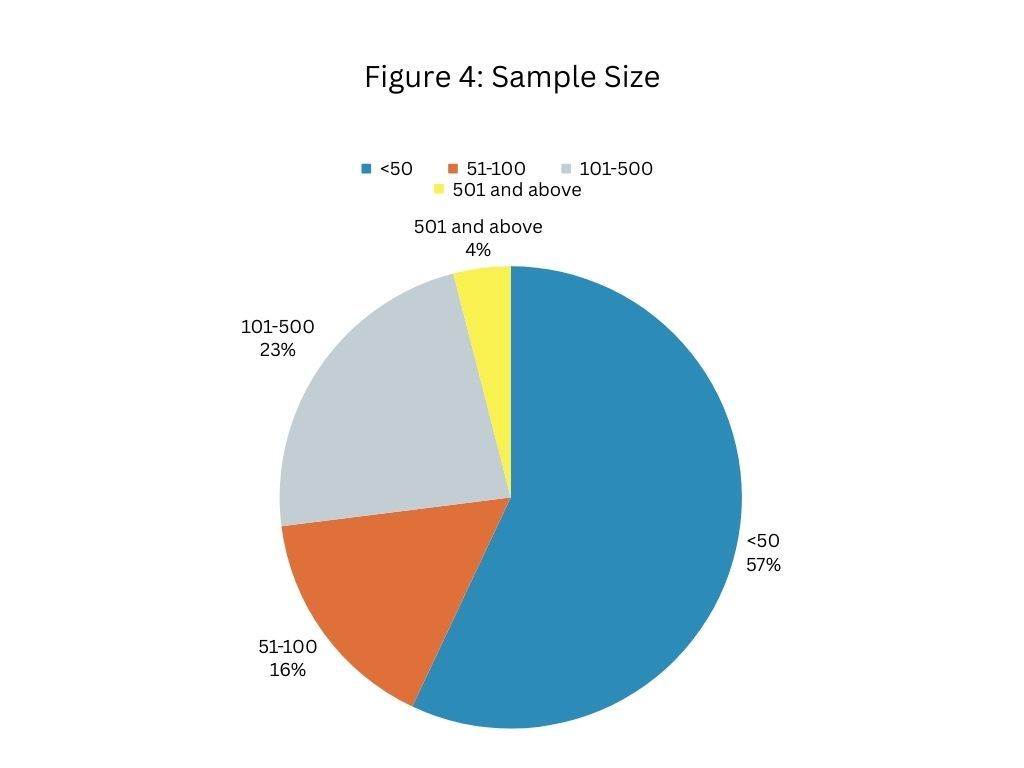
Figure 4 sheds light on the number of subjects in studies. It reflects 1) the final number of participants who completed those trials (in the case of completed studies), or 2) the target sample size (in the case of not-yet-completed studies).
Smaller Studies
Let’s take a look at some of the smaller studies (studies with a sample size of less than 50). More than half of blood sugar management studies are done with fewer than 50 participants. Take these two studies, for instance:
- Using continuous glucose monitoring (CGM) for evaluating effects of food on glucose regulation in healthy humans (6 participants)
- A study to investigate the effect of MCE on glucose and insulin response in healthy males (10 participants)
Larger Studies
Some of the larger studies (with more than 100 subjects) focus on claim areas like area-under-the-blood-glucose-and-insulin curve for type 2 diabetes patients, HbA1c, BMI in people with risk of diabetes, decreased gastrointestinal symptoms, increased time in glucose range, and decreased time in hyperglycemic range in type 2 diabetes and GI-symptomatic patients. Examples of larger studies include:
- Prevention of hypoglycemia among diabetes patients admitted to internal medicine departments with nutritional care (500 participants)
- Effect of Totum-63 on glucose and lipid homeostasis in subjects with dysglycemia (REVERSE-IT) (600 participants)
Large vs. Small Studies
A noteworthy trend has emerged—an inclination towards smaller sample sizes, meaning predominantly fewer than 50 participants. This approach may stem from logistical ease, cost considerations, or an emphasis on rapid study completion.
While smaller sample sizes may expedite studies, however, they often fall short in providing statistically significant results. This compromises the reliability and generalizability of findings, potentially impacting the credibility of the research.
This raises an imperative consideration: What is the ideal sample size for robust and impactful studies?
Ideal Minimum: 100 Subjects
A more robust approach is to consider a minimum sample size of at least 100 subjects. This threshold enhances a study’s statistical power, allowing for more reliable detection of meaningful trends and effects.
Products Analyzed in Studies
- Glucose
- Avocado
- Berberine LipoMicel (Factors Group of Nutritional Companies Inc.)
- Regular berberine hard-gels (Factors Group of Nutritional Companies Inc.)
- Microcrystalline cellulose (Slimbiotics, Clinical Research Center Kiel GmbH)
- Nutralys pea protein (Roquette Frères)
- Nutralys S85 Plus (Roquette Frères)
- Cholecalciferol 2000 IU (Diamyd Medical AB)
- AP green tea extract (Amorepacific Corp.)
- MS prebiotic (Tata Chemicals Ltd., Inquis Clinical Research (formerly GI Labs))
- TOTUM–63 (Valbiotis, Biofortis/Mérieux NutriSciences)
- EyePromise DVS (ZeaVision LLC)
- Niuchang (Inuling (Bei Jing) Science and Technology Company, Ltd.; Peking Union Medical College Hospital; Proswell Medical Corp.)
Biomarkers Analyzed in Studies
- Blood glucose level
- Insulin
- Cravings/satiety
- Full-field flicker electroretinogram (ffERG)
- Hba1c levels
- Homeostasis model assessment – Insulin
- BMI
- Plasma glucose concentration
- Serum insulin concentration
- Glycemic variability
- Body weight
- Triglycerides
- Hypertension
- Macular retinal thickness
- Visual acuity
- Neonatal glucose
- Albuminuria
- Blood pressure
- Bone volume density
*Blood glucose level, insulin, HbA1c, HOMA-IR, and serum insulin concentration stand out as crucial biomarkers extensively used in studies.
Questionnaires and Scales Used in Studies
- Daily Gastrointestinal Tolerance Questionnaire
- Quality of Life Questionnaire
- Bristol Stool Scale
- Gastrointestinal Symptom Rating Scale (GRSS)
- Depression Anxiety and Stress Scale-21
- EuroQol Five-Dimensions Quality of Life Medical Outcome Survey
- Gastrointestinal Symptom Rating Scale-LBS (GSRS-LBS)
- Hospital Anxiety and Depression Scale (HADS)
- Irritable Bowel Syndrome Quality of Life (IBSQOL) Questionnaire
- Patient Health Questionnaire 12 (PHQ 12)
- Central Sensitization Inventory (CSI)
- Gastrointestinal Symptom Questionnaire
- Social Interaction Anxiety Scale
- The Warwick-Edinburgh Mental Wellbeing Scale
- The Visual Analogue Scale Pain Intensity
*Considering the impact of elevated blood sugar levels on overall well-being, it is advisable to carefully select your questionnaire to align with the potential influence on quality of life.
Devices Used in Studies
- Sphygmomanometer
- Boso Tm-2430 Pc2
- Glycocheck Device
- Vagustim Device
- Tanita BC
- BOD POD (Air Displacement Plethysmography)
- ActiGraph Accelerometer
- Mobil-O-Graph
*The current trend involves incorporating continuous glucose monitoring (CGM) devices in research studies for precise data collection when comparing against a placebo.
References
- World Health Organization. Diabetes Facts. Updated April 5, 2023. https://www.who.int/news-room/fact-sheets/detail/diabetes
- Members of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997, 20 (7): 1183–1197.
- Dorcely, B.; Katz, K.; Jagannathan, R.; et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab Syndr Obes. 2017, 10, 345-361. DOI: 10.2147/DMSO.S100074
About the Author
Deborah Affonso oversees marketing at Vedic Lifesciences, a contract research organization (CRO) based in Mumbai, India. With a bachelor’s degree in biochemistry and botany, Affonso is entering the field with enthusiasm and a solid academic background. Although new to the industry, she is eager to apply her knowledge and embark on a career in sales and marketing within the nutraceutical and pharmaceutical sector.