Vedic Lifesciences is a contract research organization based in Mumbai, India. We recently examined clinical trials conducted in the hemp and cannabinoid space. We analyzed a total of 27 studies whose investigational product included cannabinoid or other hemp products. This report is intended to cover nutraceutical-centric studies that incorporate cannabidiol (CBD) and its derivatives like hemp and CBD oil (different extracts and forms) as the investigational product.
This report describes our findings regarding:
- The different health areas for which CBD is currently being studied
- The health claims being targeted
- Tools that have garnered attention in these studies
- The type of population that studies are being conducted in
- The overall use of CBD in the nutraceutical industry
Our findings will interest a broad range of industry professionals:
- Clinical trial managers, chief scientific officers, medical directors, and other personnel involved in designing or executing both preclinical and clinical studies
- Marketing and communications managers who wish to explore the CBD market to identify new health claims/benefits to create unique selling points for their products
- R&D decision makers who would like to explore the inclusion of CBD or any of its derivatives in their products’ development
- CEOs, general managers, and senior directors who can influence decisions regarding study design and research budgets
- Regulatory and quality chiefs who want to understand the science that’s been conducted on CBD in the last five years
By attempting to identify the innovative health claims being investigated for specific forms of CBD, this report aims to help R&D teams formulate their functional ingredients or branded supplements using various CBD types and doses, and design unique trials with trending objectives. It also aims to help company managers make informed decisions about marketing their products and planning their growth and to provide companies looking to enter the CBD market with more information on results of recent clinical and preclinical studies.
In terms of our search methods, we used the clinical trial registry www.clinicaltrials.gov instead of a search engine like PubMed, which would only have yielded trends from published studies initiated more than two years ago. By contrast, a clinical trial registry provides more current data, including data on studies not yet completed.
Here are the filters we used in our analysis:
- Study date range: Studies starting January 1, 2018, and ending December 31, 2022 (a 5-year period)
- Search terms: dietary supplement cannabinol, Cannabis sativa, cannabis oil, cannabidiol oil, hemp extract, hemp seed oil, CBD oiI, hemp oil, cannabidiol, hemp, cannabinoid, CBD. (Duplicates were removed, and studies not containing these keywords, or any drug trials, were excluded from the report, making this the limitation of the analysis.)
Health Area
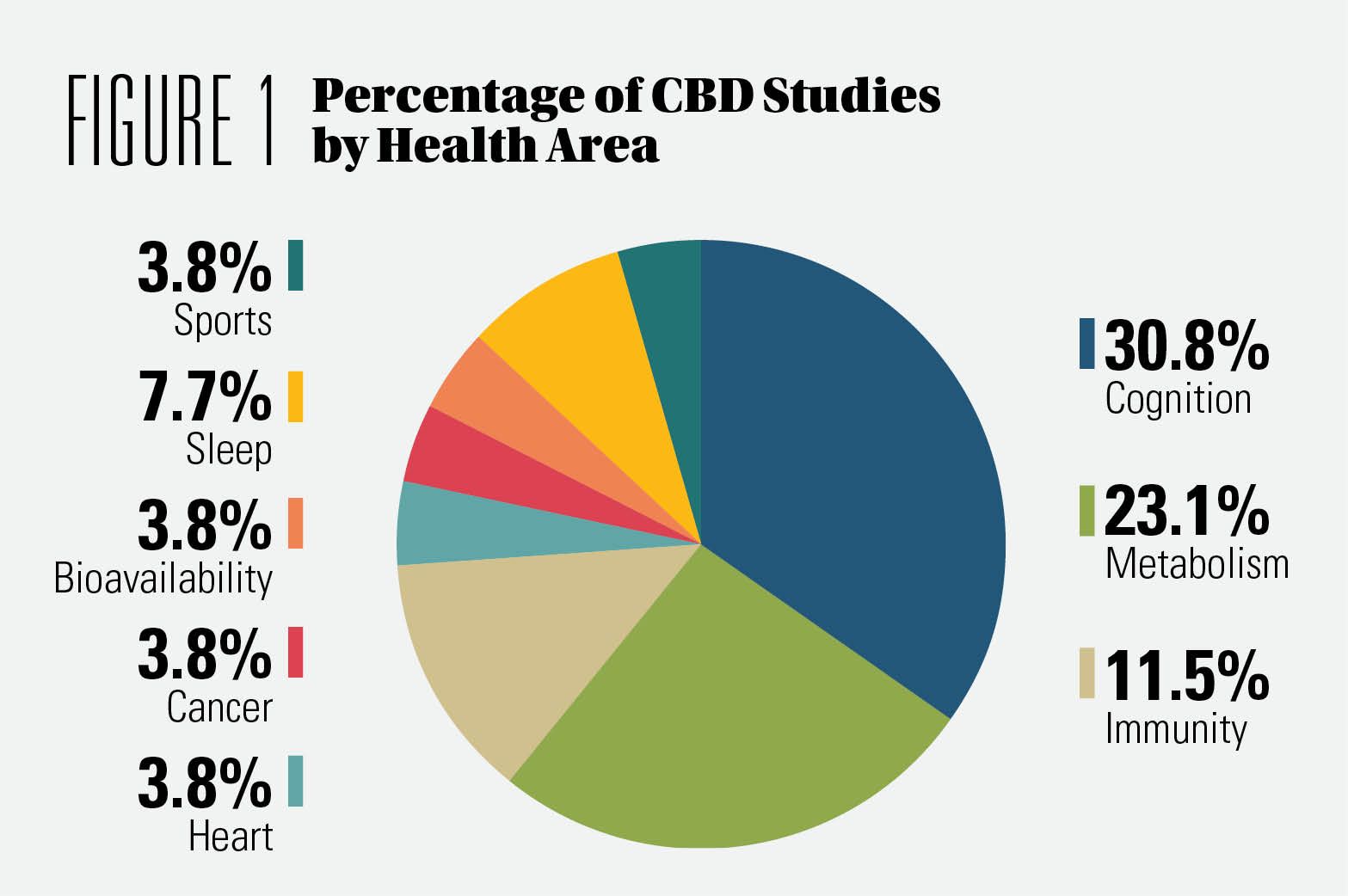
Which health concerns is CBD being studied for?As Figure 1 shows, the majority of CBD research is on cognitive support. The primary objective of more than one-third of the studies we analyzed was some aspect of cognitive health. Secondary outcomes included changes in sleep, quality of life, physical activity, and behavioral patterns at baseline and end of the study. Figure 1 also shows that CBD is now being studied for metabolic health (weight management, glucose levels, lipid profiling).
Study Endpoints
Which study endpoints are these CBD studies focusing on? Table 1 provides a detailed look at some of the most common endpoints for health concerns ranging from cognition and metabolism to immunity, heart health, joint health, sleep, sports performance, and more.
Tools and Biomarkers
Which tools and biomarkers did these CBD studies use? Table 2 provides a detailed look at some of the most common tools and biomarkers used in studies for health concerns ranging from cognition and metabolism to immunity, heart health, joint health, sleep, sports performance, and more.
Sample Size
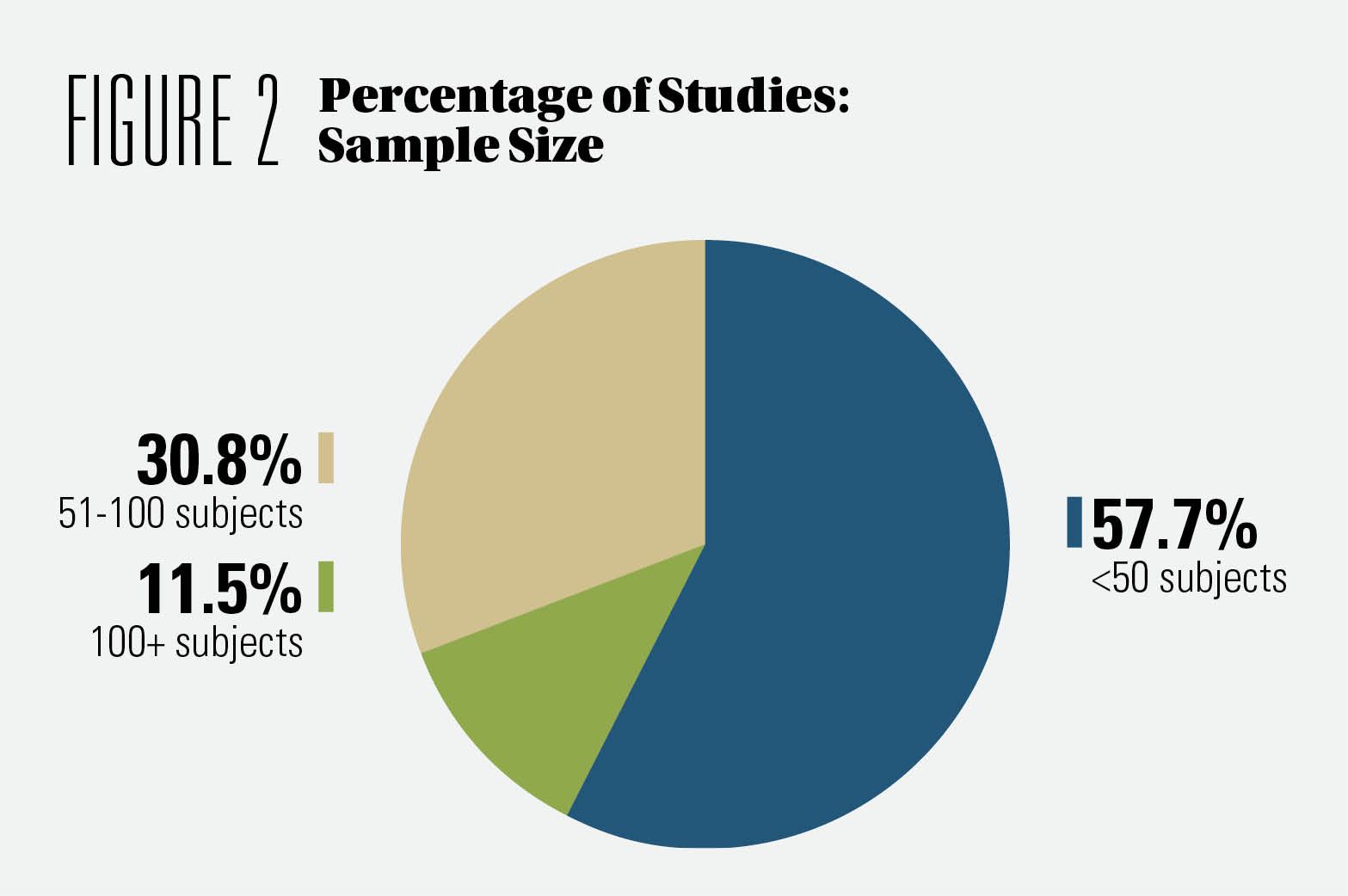
Interest in CBD has led to more than 150 proof-of-concept studies. Approximately 58% of the studies we analyzed had a sample size of fewer than 50 subjects. (See Figure 2) More companies and research institutes are initiating proof-of-concept/pilot studies on CBD. This would be a space to watch out for. Large-sample-size studies should follow suit based on the data obtained in the studies.
Completion Status
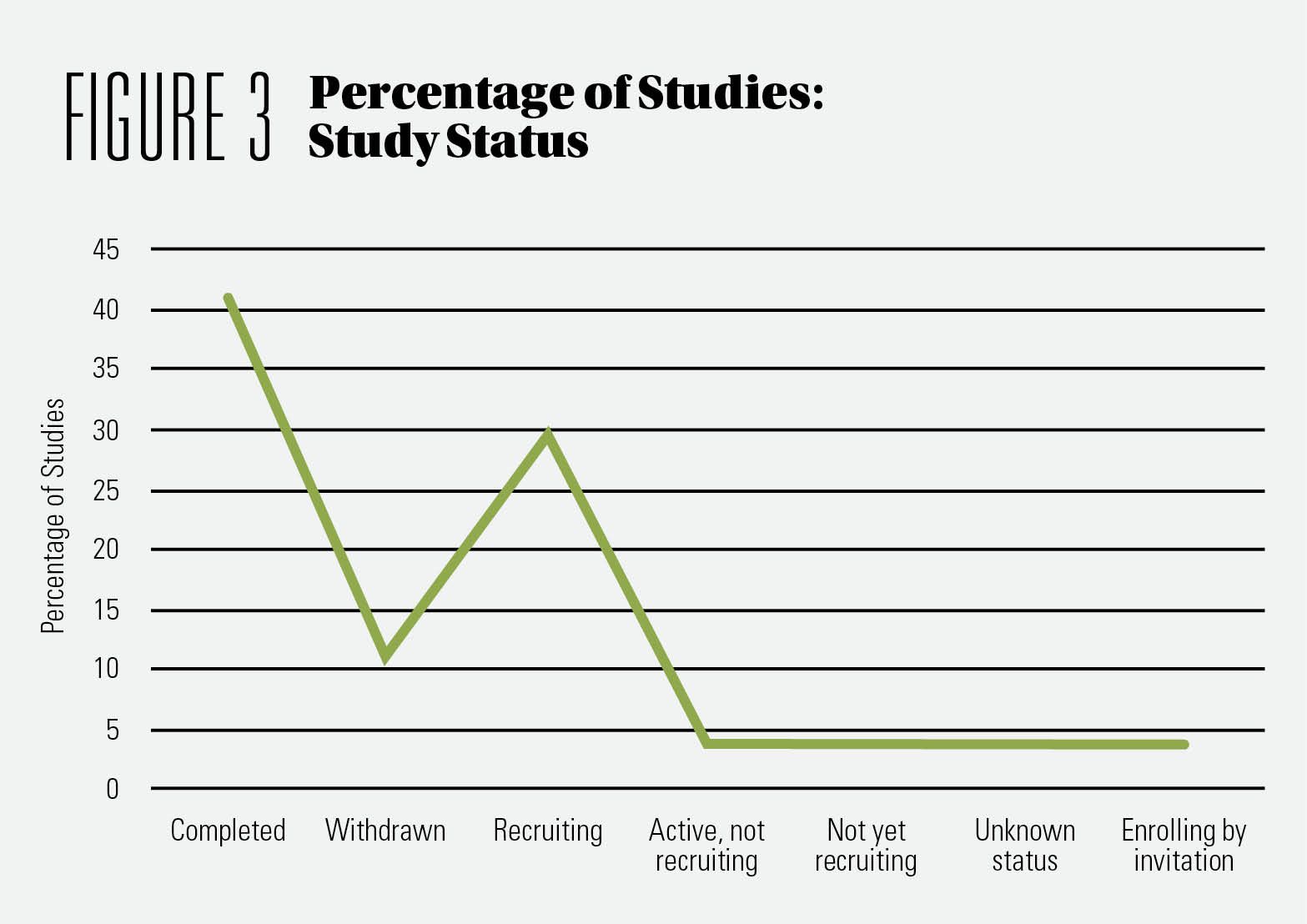
Interestingly, while more than 42% of the studies we analyzed had been completed, no results were highlighted in the clinical trials registry. (See Figure 3) Another fact we noticed is that close to 31% of the studies are currently recruiting. This means that the research on CBD has gained momentum in the last five years. Finally, both types of studies—completed and in progress—encompass a range of health areas, meaning several health claims are substantiated.
Study Duration
More than 53% of the total studies we analyzed had a duration of less than 12 months—with some still in the recruitment phase in terms of their completion date. (See Figure 4) This fact, combined with a trend observed of smaller study sample sizes, indicates that several companies are interested in faster substantiation of their health claims. This may then lead to large-sample-size studies in the next couple of years and faster market inclusion of their products.
Safety vs. Efficacy Studies
While there are more of proof-of-concept studies, close to 77% of studies are efficacy studies rather than safety studies. This indicates that companies may be directly jumping into substantiating health claims rather than performing the quintessential safety assessment of different extracts of CBD.
Other Interesting Insights
Interesting Takeaways from the Studies We Assessed
- None of the studies we analyzed were conducted on children below the age of 18 years.
- Most of the studies were conducted on both men and women. There was one study that focused only on women. The study aimed to understand the effect of different forms of CBD and their doses in 1350 women. The study was sponsored by Rae Wellness and was one of the largest-sample-size studies done with CBD.
- Both core CBD companies and diverse-portfolio companies have jumped into clinical studies on one CBD form or another. Companies like Prima (study on anxiety), Caliper Foods (study on metabolism), YEAHHH! Baby LLC (study on joint pain), Futureceuticals and Folium Biosciences (study on cognitive support), Gaia Herbs (study on Tmax, absorption), Beam (study on sleep), and others have diverse health areas and diverse objectives to substantiate.
- Two of the most interesting studies done explored the effect of CBD extract (combined with other ingredients like algae) in the prevention or reduction of COVID-19 symptoms, including hospitalization, and those experiencing symptoms of Post-Acute COVID Syndrome (PACS). This also could have implications for other post-disorders or diseases symptoms improvement and subsequent quality of life improvement.
- Daily beverage intervention was a notable delivery method used in CBD testing by Ocean Spray in conjuction with universities to understand changes in health and fitness, fatigue, stress, calmness, quality of life, cognitive function, ability to maintain focus, sleep quantity, and sleep quality.
- CBD studies are more likely to have unique clinical trial designs with unique endpoints and use a higher percent of tools and questionnaires. The focus on biomarkers has shifted with the inclusion of trending protocols.
When we analyzed the studies—excluding those whose status was either “withdrawn” or “unknown,” two studies caught our attention, for different reasons.
First, in the study NCT05108220, 1350 female subjects aged 24-44 (working population) were recruited in order to investigate the effect of CBD on subjects’ anxiety levels. The dose-ranging study included CBD oil and capsules with 0.0%-0.3% THC content. In-Home Usage Tests (IHUT) were done to understand the different CBD compounds’ effects on anxiety in women.
Second study NCT04777981 was interesting. The focus was the effects of CBD, in combination with red algae, on symptomatic improvement in COVID-19 patients.
Other key takeaways is that interest in CBD’s benefits is not just limited to cognition. While cognitive support is a known area of CBD research, interest in other health areas like heart health (hypertension), cancer (symptomatic improvement), sleep quality, immune health, and metabolic health demonstrate broad interest in CBD inclusion in the nutraceutical industry.
Recommendations for Research from a Regulatory Perspective
According to certification and testing firm FoodChain ID, both preclinical and clinical studies are mandated for regulatory submission for any novel food. These include the bacterial reverse mutation test, in vitro micronucleus test in mammalian cells, and a 90-day study. Also for CBD, global standardized guideline tests to be done would be OECD 451,452, 443, and 422.
After preclinical studies are concluded, a safety study in healthy individuals is needed. Safety can be analyzed by assessing the comprehensive metabolic panel, which tests the levels of electrolytes and kidney and liver parameters. Tests can also include lipid profile, hormone profiling for men and women, semen analysis, and inflammatory biomarkers.
Another good practice is understanding the sleep quality, cognitive health, and gut parameters using questionnaires. This data can then be studied in conjunction with the safety tests to draw final conclusions about the effect of CBD in humans.
About the Author
Palak Vaval is Senior Associate – Business Development, at Vedic Lifesciences, a contract research organization (CRO) based in India. She caters to North & South American nutraceutical companies’ claims substantiation requirements. With a Master’s degree in Life Sciences (Biotechnology), Vaval has close to a decade’s worth of experience in sales and digital marketing with respect to the food and nutraceutical industry.